Several properties related to the atomic bonds and angles between them can be computed using I.S.A.A.C.S.
In I.S.A.A.C.S. the existence or the absence of a bond between two atoms i of species α and j of species β is determined by the analysis of the partial gαβ(r) and total g(r) radial distribution functions.
Precisely the program will consider that a bond exists if the interatomic distance Dij is smaller than both the cutoff given to desribe the maximum distance for first neighbor atoms between the species α and β, Rcutαβ (often the first minimum of the partial radial distribution function gαβ(r) ), and the first minimum of the total radial distribution function, Rcuttot.
I.S.A.A.C.S. allows the user to specify both Rcutαβ and Rcuttot to choose an appropriate definition of the atomic bonds to described the system under study.
When atomic bonds in a model are defined properly other structural characteristics can be evaluated, as follows:
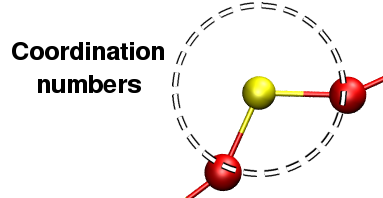
Average first coordination numbers
I.S.A.A.C.S. computes total as well as partials coordinations numbers.
Figure 1: Coordination numbers.
Individual atomic neighbor analysis
I.S.A.A.C.S. computes the fraction of each type of first coordination spheres occurring in the model.
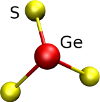
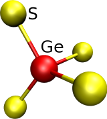
The presence of of structural deffects can lead to a wide number of local structural environments, figure [Fig. 2] illustrates the differents first coordination spheres that can be found in a GeS2 glass.
Figure 2: Illustration of several coordination spheres that can be found in glassy GeS2.
Proportion of tetrahedral links and units in the structure model
Often the structure of a material is represented using building blocks.
One of the the most frecuently occuring building blocks are tetrahedra.
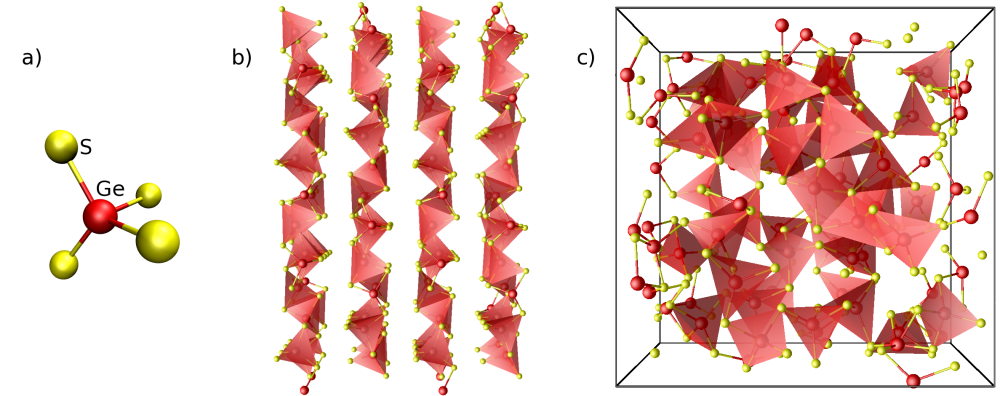
Figure [Fig. 3] shows a model of GeS2 materials using GeS4 tetrahedra as building blocks.
Figure 3: Illustration of the presence of GeS4 tetrahedra in the GeS2 material's family.
a) GeS4 tetrahedra, representations b) of the α-GeS2 crystal and c) of the GeS2 glass using tetrahedra.
I.S.A.A.C.S. computes the fraction of the differents tetrahedra in materials, the distinction between these tetrahedra being made on the nature of the connection between each of them.
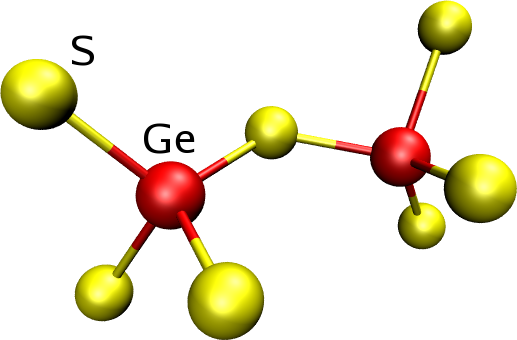
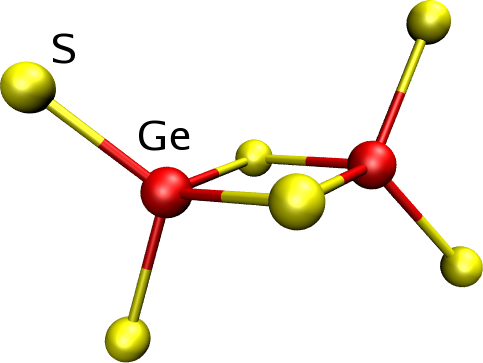
Tetrahedra can be linked either by corners [Fig. 4] or edges [Fig. 5], I.S.A.A.C.S. computes the fraction of atoms forming tetrahedra as well as to the fraction of linked tetrahedra.
|
Figure 4: Corner sharing tetrahedra. |
Figure 5: Edge sharing tetrahedra. |
Distribution of bond lengths for the first coordination sphere
Figure 6: Nearest neighbor distances distribution.
Angles distribution
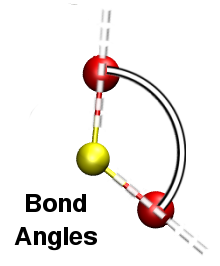
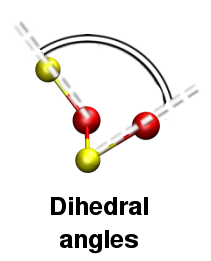
Using I.S.A.A.C.S. it is very easy to compute bond angles [Fig. 7] and dihedral angles [Fig. 8] distributions:

|
|